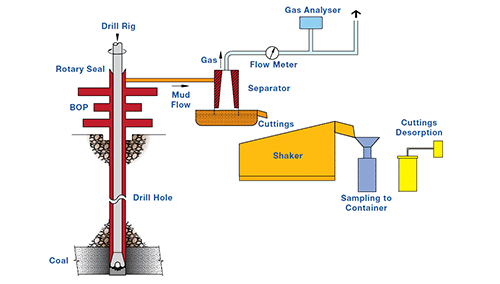
The gas itself may be a danger either because it is toxic, such as carbon dioxide or hydrogen sulphide, because it may lead to an asphyxiating atmosphere or because it may produce an explosive atmosphere within the workings.
Gas may be stored in rock within the pore or fracture space. Here the key parameters are the pressure of the gas and the volume of that space. It may also be stored as a liquid. For example carbon dioxide becomes a liquid if the pressure exceeds 7.4 MPa and the temperature is below 31oC. Gas may also exist in solution. In petroleum reservoirs there are frequently changes of phase and solution during production. In carbonaceous rock such as coal and shales the gas is also stored by a process described by the all encompassing term, sorption. Sorption principally involves bonding of the gas by Van der Waal’s forces to the hydrocarbons in the rock. It may also involve chemisorption.
In dealing with gas in rock the key parameters that need to be considered are the amount of gas, the pressure at which it is stored and the type of gas. The way in which gas is transported through the rock is also important – is it by Darcy flow or by some form of diffusion?
In summary the key information that is needed in dealing with the gassy rocks are:
The manner in which the gas is stored is also important as it will affect the process of release.