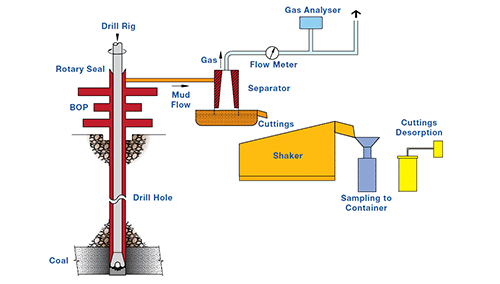
The first, and more common, is the laboratory isotherm where the sample has gas forced into it and then is desorbed. The second is the native sorption isotherm where a core sample is desorbed in a series of pressure steps from the gas content it has when it reaches surface. The Native isotherm has the advantage that it involves testing with the gas within the sample and at the sample moisture content.
The laboratory isotherm has several complications. Most of these are related to the types of gas and the amount of water within the sample. Higher moisture contents lead to reductions in the isotherms. As it is only possible to test with a single gas at a time the recreation of isotherms with multiple gas types then becomes a mathematical process with an outcome that is not experimentally derived.